Advantages and Disadvantages of Different Treatment Methods in Achondroplasia

Who conducted the study?
The study was conducted by Wiktoria Wrobel, Emilia Pach and Iwona Ben-Skowronek from the Metabolic Laboratory, Department of Paediatric Endocrinology and Diabetology with Endocrine, Medical University in Lublin, Poland.
What was the purpose of the study?
The aim of this study was to analyze the pharmacological therapeutic methods in achondroplasia, including the advantages and disadvantages of all drugs, both those currently used and those in different phases of clinical trials that have been demonstrated in human and animal studies.
This article includes the potential impacts of drugs on achondroplasia symptoms other than short stature, including their effects on spinal canal stenosis, the narrowing of the foramen magnum and the proportionality of body structure. Addressing these effects could significantly improve the quality of life of patients, possibly reducing the frequency and necessity of hospitalization and painful surgical procedures, which are currently the only therapeutic options used. The criteria for a good drug for achondroplasia are best met by recombinant human growth hormone at present and will potentially be met by vosoritide in the future, while the rest of the drugs are in the early stages of clinical trials.
Methods of treatment analyzed:
The methods for treating achondroplasia can be grouped into surgical and pharmacological therapies. Surgical intervention consists of lengthening the lower limbs using an Ilizarov apparatus or monolateral external fixator and entails multiple procedures and possible serious complications.- C-Type Natriuretic Peptide in Achondroplasia Treatment
- C-Type Natriuretic Peptide Analog: Vosoritide (BMN-111)
- Prodrug, Prolonged-Release C-Type Natriuretic Peptide: TransCon CNP
- Recombinant Human Growth Hormone (rhGH)
- Tyrosine Kinase Inhibitor (TKI): Infigratinib
- Soluble FGFR3 (TA-46): Recifercept
- Vofatamab (B-701)
- Meclizine
- Statins
- Parathyroid Hormone (PTH) and Parathyroid Hormone-Related Peptide (PTHrP
- FGFR Inhibitor: ASP5878
- Aptamer RBM-007
Results
Table 1. Classification of drugs according to the target of action
| Target of Action | Drug Name |
| FGFR3 | sFGFR3 (recifercept) |
| Inhibitors of HMG-CoA (statins) | |
| Tyrosine kinase inhibitor (infigratinib) | |
| FGFR 1–4 inhibitor (ASP5878) | |
| FGFR3 antibody (vofatamab) | |
| NPR-B | CNP analogue (vosoritide) |
| Sustained-release CNP prodrug (TransCon CNP) | |
| MAPK pathway | Meclizine |
| Chondrocyte nucleus | rhGH |
| PTH/PTHrP |
| Abbreviations: CNP—C-type natriuretic peptide; FGFR—fibroblast growth factor receptor; HMG-CoA—3-hydroxy-3-methylglutaryl-coenzyme A reductase; NPR-B—natriuretic peptide receptor B; MAPK—mitogen-activated protein kinase; PTH—parathyroid hormone; PTHrP—parathyroid hormone-related peptide; rhGH—recombinant human growth hormone; sFGFR3—soluble fibroblast growth factor 3 receptor. |
Table 2. Benefits of different drug therapies in achondroplasia
|
Drug Name
|
Benefits for People
|
Benefits for Animals
|
|
|
Clinically used drugs
|
|
|
Recombinant human growth hormone (rhGH)
|
Possibly better growth pattern in children with achondroplasia, especially in combination with l-thyroxine and surgical elongation of tibia and/or femur
|
Possibly better growth velocity with variable rather than continuous drug administration
|
|
|
Drugs in different phases of clinical trials
|
|
|
C-type natriuretic peptide (CNP) analog: vosoritide
|
Treatment targets underlying molecular pathogenesis, increasing growth velocity and height Z-score
More proportional growth Resistance to natural endopeptidase No serious side effects |
Increase in axial and appendicular skeleton growth
Increase in the hypertrophic zone in tibial growth plates Widening of lumbar vertebral openings |
|
C-type natriuretic peptide prolonged-released: TransCon CNP
|
Long half-life, about 90 h
Resistance to natural endopeptidase Prevents adverse cardiovascular effects because of long-release form Phase II of research in progress NCT04085523 |
Increase in body and tail length in monkeys
No adverse effect on bone quality Increase in the width of the proliferative zones in the proximal tibia |
|
Infigratinib
(NVP-BGJ398) |
No data
Phase II of research in progress NCT04265651 |
Increases the growth of long bones, axial and craniofacial skeleton
Increases the size of foramen magnum Correction of spinal stenosis Ameliorates the defective differentiation of the chondrocyte |
|
Soluble recombinant human fibroblast growth factor receptor 3 (soluble FGFR3): recifercept
|
No data
Phase II of research in progress NCT04638153 |
Reduces mortality
Restores skeletal bone growth Increases cortical bone thickness Decreases spinal and skull deformities Enlargement of pelvic bone Corrects metabolic alteration (helps with atypical obesity) |
|
Vofatamab (monoclonal antibody specific for FGFR3)
|
No data
|
No data
|
|
Meclizine
|
Administered orally
No data |
Increases the growth of long bones, axial and skull lengths
Ameliorates short stature Increases trabecular thickness |
|
Statin
|
Ambiguous data
|
Ambiguous data
|
|
ASP5878
|
Administered orally
|
Increases the growth of long bones
Elongates the length of the cranial base Increases thickness of growth plate cartilage |
|
Parathyroid hormone (PTH)
|
Causes proper development of cartilage tissue
Increases proliferation and differentiation of chondrocytes and mesenchymal cells, extracellular matrix synthesis |
Positive effect on growth velocity, similar body length to the rest of the healthy litter
Retardation of premature fusion of the skull synchondrosis Inhibition of FGFR3 activation |
Table 3. Drawbacks of different drug therapies in achondroplasia
|
Drug Name
|
Drawbacks for People
|
Drawbacks for Animals
|
|
|
Clinically used drugs
|
|
|
Recombinant human growth hormone (rhGH)
|
Theoretical possibility of the appearance of acromegaly signs, increase in foramen magnum narrowing and spinal cord compression, but no conclusive evidence
Ineffective in the case of deformation of the limbs and spine Requires daily subcutaneous injections |
Increases body mass
|
|
|
Drugs in different phases of clinical trials
|
|
|
C-type natriuretic peptide (CNP) analogue: vosoritide
|
Mild side effects: transient changes in blood pressure
Requires daily subcutaneous injections |
Transient, mild hemodynamic effects
|
|
C-type natriuretic peptide prolonged-released: TransCon CNP
|
No data
Phase II of research in progress NCT04085523 |
Dose-dependent lowering of blood pressure in mice but not in monkeys
|
|
Infigratinib
(NVP-BGJ398) |
Administered in injections
No data Phase II of research in progress NCT04265651 |
No effect on the defect in the structure of long bones
Not found |
|
Soluble recombinant human fibroblast growth factor receptor 3 (soluble FGFR3): recifercept
|
Administered in injections
No data Phase II of research in progress NCT04638153 |
No effect on the trabecular bone
Effects are mediated only through FGF-dependent pathway No signs of toxicity Preserved fertility |
|
Vofatamab (monoclonal antibody specific for FGFR3)
|
No data
|
No data
|
|
Meclizine
|
Phase I completed: no signs of toxicity were found
|
No effect on the area of foramen magnum or lumbar spinal canal
Cumulative effect of 20 mg/kg—toxicity: ineffective bone growth |
|
Statin
|
Ambiguous data
|
Ambiguous data
|
|
ASP5878
|
Hyperphosphatemia
Retinal detachment Diarrhea Elevated alanine transaminase |
Slight atrophy of the corneal epithelium
|
|
Parathyroid hormone (PTH)
|
Unknown long-term effects, need for further studies on the safety profile
|
No data
|
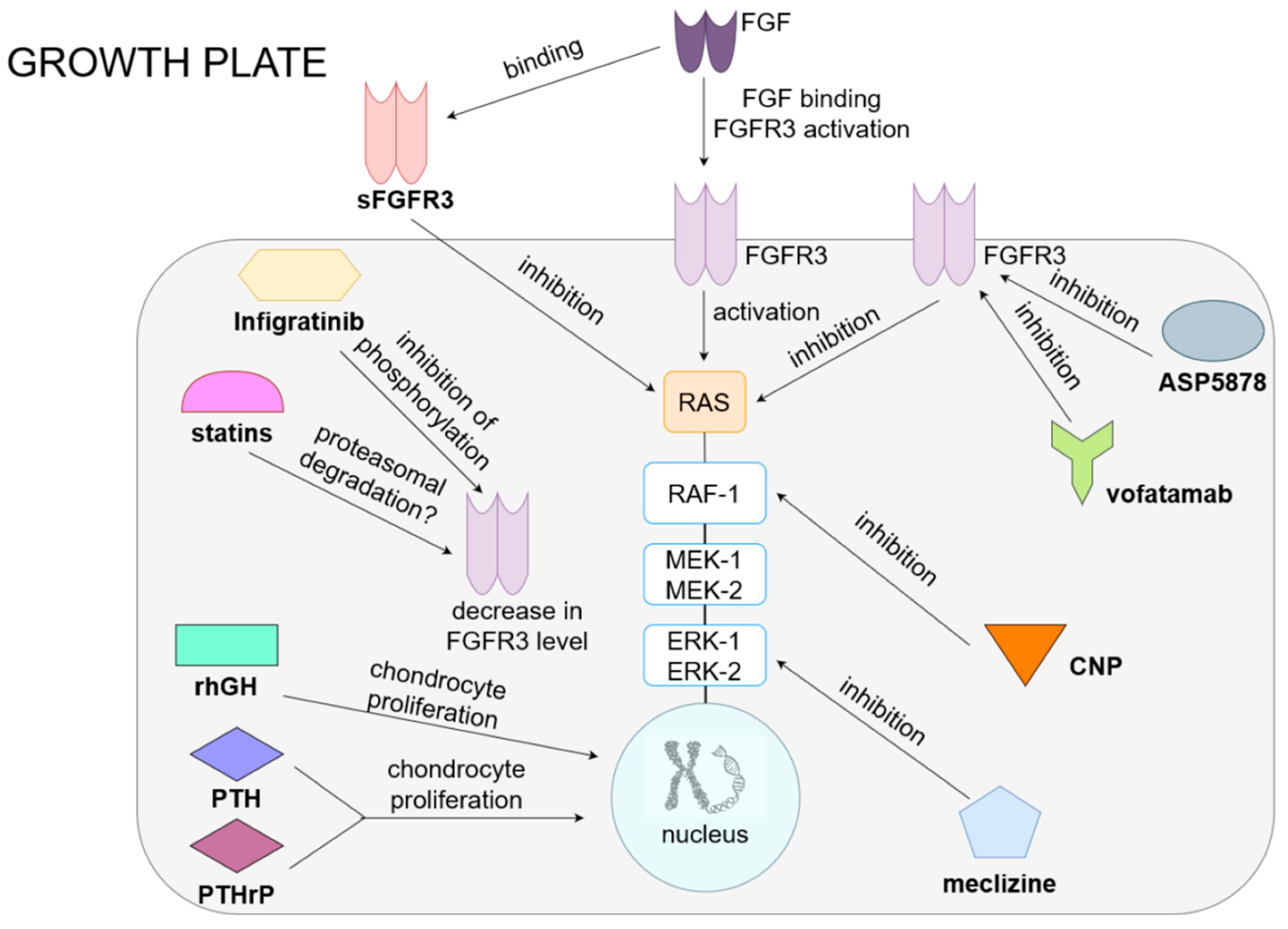
Figure 1. Current and potential treatments for achondroplasia.
In the normal growth plate, FGF acts on FGFR3, causing the activation of RAS and the MAPK pathway (RAF, ERK and MEK), resulting in the inhibition of chondrocyte proliferation and differentiation and a reduction in extracellular matrix synthesis. Drugs for achondroplasia include preparations that influence the function of the FGFR3 receptor and MAPK pathways and directly act on chondrocytes. The first group includes, among others, drugs that inhibit the receptor (ASP5878, which is an inhibitor of FGFR3, and vofatamab, an anti-FGFR3 antibody), as well as drugs that reduce the concentration of FGFR3 (infigratinib, which inhibits receptor phosphorylation, or statins, potentially causing proteasomal degradation of this molecule). The drug that competes with FGFR3 for a ligand is sFGFR3, a soluble receptor that, by binding to FGF, reduces its effect on the growth plate. Drugs that inhibit the MAPK pathway are CNP analogs, which inhibits RAF-1, and meclizine, which inhibits ERK. By acting on FGFR3 or MAPK, all of these drugs consequently increase the proliferation and differentiation of chondrocytes, improving bone elongation growth. RhGH and PTH or PTHrP have a direct stimulating effect on the proliferation and differentiation of chondrocytes.
Conclusions
Current data shows that finding a cure for achondroplasia is the subject of very intensive research by teams across the globe. For now, rhGH and vosoritide studies are more advanced. However, even if these treatments reach widespread use, they will not cure all the symptoms associated with achondroplasia. They can provide the benefit of increasing bone length, but their effects on disproportionality, the axial skeleton and the foramen magnum, have not been confirmed. Each of these entails further complications that leave their mark on the daily lives of people with achondroplasia.
The ideal drug for achondroplasia should be:
- small in size, to readily penetrate the growth plate
- be specific for FGFR3 and effectively inhibit its signaling pathway
- as the therapy is long term, its production costs should be as low as possible, and the form of drug administration should be easy and acceptable for a pediatric patient
- side effects should be minimized to the level of dose tolerance.
The criteria for a good drug for achondroplasia are best met by recombinant human growth hormone at present and will potentially be met by vosoritide in the future, while the rest of the drugs are in the early stages of clinical trials.
Read the full review here.


