Achondroplasia Natural History Multicenter Clinical Study
2016-02-21Research
This study not only means to understand the natural history of achondroplasia (what complications occur, how often, average life span, etc.), but also aims to build a global patient registry. What is the purpose of a patient registry? Know more about it here.

Vosoritide Proceeding to Phase 3
2016-02-02Research
What are the next steps for BMN-111? Here you can find a timetable for what is planned for this rdug in development.
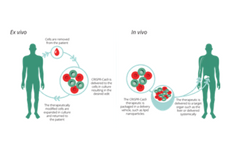
To better understand CRISPR-Cas9
2016-01-03Research
New treatments using the CRISPR/Cas9 gene editing tool are being developed. In order to understand how these would work here is a short vídeo and article about this possible excision of the mutation and substitution with the non-mutated gene.
Cutting Off The Mutation
2016-01-01Research
CRISPR/Cas9 is powerful new gene editing tool, making development of new treatments easier, faster and cheaper. It's potential for use in humans is currently under study, but how does it work exactly, and why is it so versatile? Learn more about this revolutionary technology here.

BioMarin investors page announces
2015-11-25Research
BioMarin intends to move into Phase 3 study discussions on the next stage of development with health authorities with a dose of 15 micrograms per kilogram daily. Also, a new cohort, designed to test a new dose regimen has completed enrollment for the Phase 2 study.

New cohort revealed for phase 2 of BMN-111
2015-10-19Research
A new cohort for the phase 2 clinical trial for BMN-111 has been revealed, learn more about it here.


