BioMarin Announces Positive Final Results for Vosoritide - Phase 3
16th Dec 2019
The primary endpoint of the study is the change in growth velocity from baseline over one year in children treated with vosoritide compared to placebo. A wide range of secondary and exploratory endpoints include anthropometric measures such as height Z-score, body and limb proportionality and joint geometry; biochemical, biomarker and radiological assessments of bone growth and health; and evaluations of health-related quality of life (HRQoL), developmental status, and functional independence.
These additional endpoints address the overall impact vosoritide has on achondroplasia and will continue to be evaluated in an ongoing open-label extension study where all subjects receive active treatment.2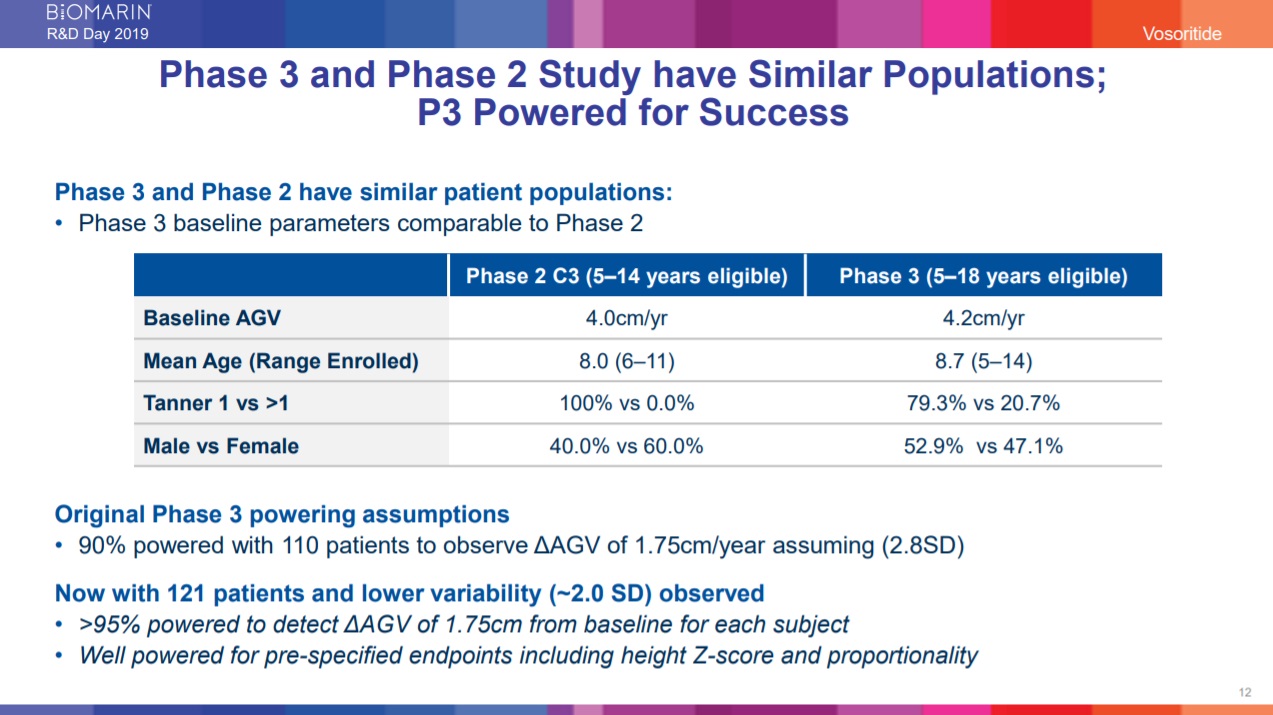 Figure 1. BioMarin R&D Day - 14th November 2019
Figure 1. BioMarin R&D Day - 14th November 2019
Based on the results, BioMarin plans to have pre-submission meetings with Health Authorities Planned for the first half of 2020 to discuss Marketing Applications for Market authorization.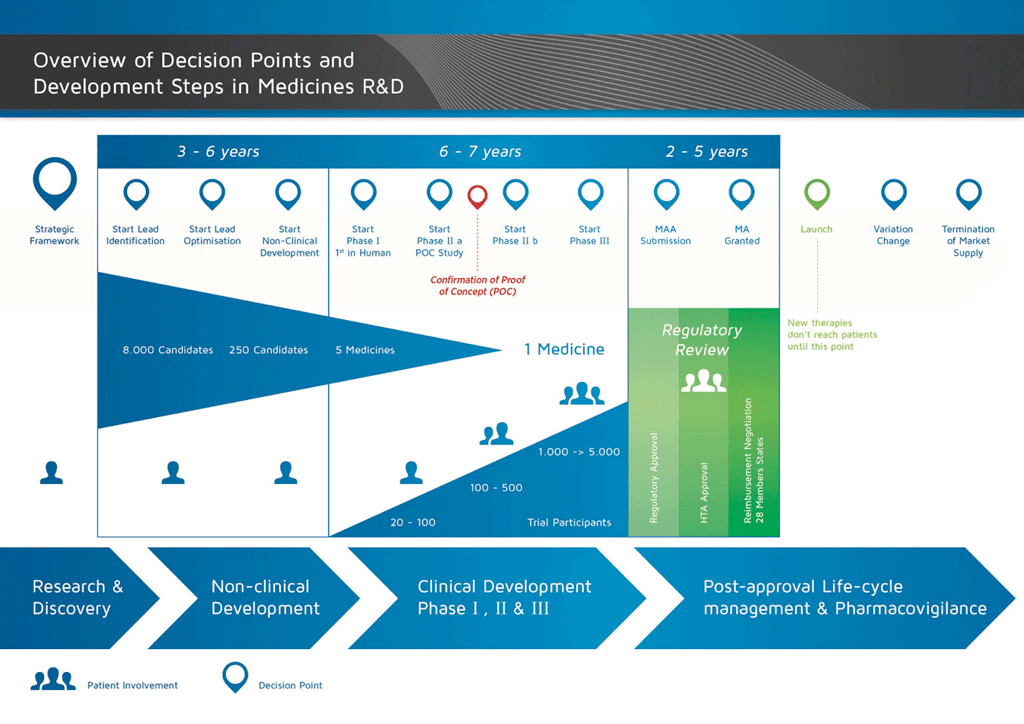
Figure 2. Marketing Authorisation Applications, EUPATI
Medicines development process is a long journey. The ultimate goal of any development process of a new medicinal product, as vosoritide, is market approval, called Marketing Authorisation (MA).3
A market application is an application submitted by a drug manufacturer/pharma company seeking permission to bring a medicinal product (for example, a new medicine or generic medicine) to the market. The main Health Authorities are EMA (Europe), FDA (USA) and PDMA (Japan)
Market Application Authorization (MAA) is part of the official procedure before the Committee for Medicinal Products for Human Use of the European Commission. In the USA, the equivalent process is called New Drug Application, conducted by FDA.
So, if vosoritide development keeps being positive, there is still a long process before it becomes available for children outside the clinical trial. And it may not become available in all countries but only in those BioMarin submits a market application.
Resources:
1. BioMarin - Placebo-controlled phase 3 vosoritide
2. Seeking Alfa
3. EUPATI - Marketing Authorisation ApplicationsMarketing Authorisation Applications


