The International Society of Skeletal Dysplasia 2017 meeting report
The International Skeletal Dysplasia Society meeting was held in Bruges between the 20th and 23rd September 2017, with the presence of world-renowned geneticists and clinicians with interest in skeletal dysplasias; also some pharmaceutical companies and patients representatives from all around the world were present.
The companies that were present and that are currently developing medicines for achondroplasia were, by alphabetic order: Ascendis Pharma, BioMarin, and Therachon.
ALPE Foundation has a report of this meeting, where the most relevant information is shared.
To add some clarification on the concepts regarding the Meclozine representation by Hiroshi Kitoh, that presented "Oral administration of meclozine for the treatment of short stature in achondroplasia"
His team observed that cutting-off the FGFR3 signaling in-vitro promoted (produced) longitudinal bone growth in transgenic ACH mice (mice that were genetically changed to be born with the ACH mutation). The team has been investigating which is the optimal dose of meclozine for the treatment of short stature in ACH for further clinical application in children.
| Credits: Conversantbio.com |
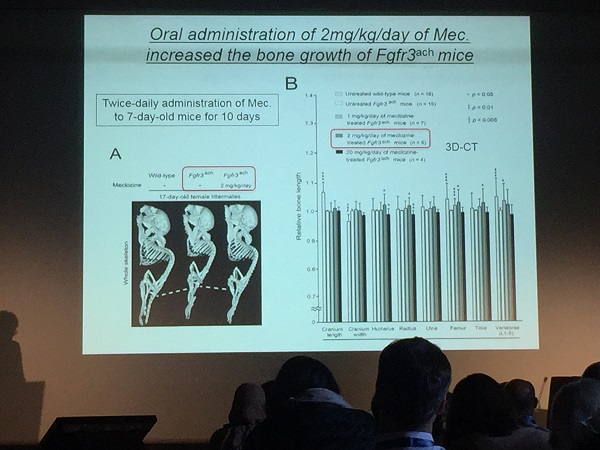
For this study, the team administered orally 1, 2 or 20 mg/kg/ day of meclozine to 7-day-old FGFR3 ach mice for 10 days. The body lengths were measured during the treatment periods and at the end of the treatment, the mice were subjected to micro-computed tomography (micro-CT) scans for calculating the bone length and bone volume.
The pharmacokinetic analyses demonstrated that peak drug concentration (Cmax) of 2 mg/kg of meclozine to mice was similar to those of 25 mg/body to human, which is a clinical usage for anti-motion-sickness. The body lengths of FGFR3 ach mice were increased by oral administration of 1 or 2 mg/kg/day of meclozine and the bone volume and trabecular bone quality were improved by meclozine treatment. Treatment of 20 mg/kg/day of meclozine, however, showed no positive effects on bone growth of mutant mice.
Kitoh finished saying that they confirmed a preclinical proof of concept for applying meclozine for the treatment of short stature in ACH, although toxicity and adverse events associated with long-term administration of this drug should be examined. Also, the team is aiming to start a phase 1 clinical trial in Nagoya, Japan in children with ACH, between the end of 2017 and beginning of 2018. The study design is still not disclosed.
Metabolic studies on Achondroplasia
But one of the most relevant moments during ISDS was a study presented by Celine Saint-Laurent, a young researcher working in Elvire Gouze's team in Nice, France (and in collaboration with Therachon). In her presentation, Celine showed that there is a link between the FGFR3 mutation and the metabolic complications related to achondroplasia.
Prior to this study in a mouse model with ACH, Celine firstly conducted a retrospective longitudinal study in children and adolescents with achondroplasia carrying the most frequent mutation of achondroplasia: the G380R FGFR3 mutation. Anthropometric, densitometric measures and several blood parameters were recorded from birth onward during follow up visits and compared between three age groups ranging from [0-3], [4-8] and [9-18] years old.
Celine's study confirmed that there are metabolic disturbances in achondroplasia patients, that are unexpectedly not associated with classical obesity complications. Indeed, the data gathered showed that while patients with achondroplasia usually develop abdominal obesity, this does not correlate with an increase of typical risk factors of obesity such as high glucose, insulin or lipid levels and that they do not appear to develop diabetes.
In the mouse model study, Celine injected the mice with Flag-soluble FGFR3 (sFGFR3) during the mice growth period and could confirm that the bone growth was restored and the metabolic deregulations were corrected with the administration of soluble FGFR3. The researchers concluded that sFGFR3 proved to be a promising treatment for achondroplasia by restoring bone growth and also by preventing the metabolic deregulations and the development of obesity.


