TA-46 Mechanism Of Action
TA-46 is Therachon´s approach to achondroplasia, showing promising pre-clinical results.
Achondroplasia is caused by a single point mutation in the gene that encodes for Fibroblast Growth Factor Receptor 3 (FGFR3), causing it to be constitutively activated without the need of the signaling molecules usually needed to activate it to halt growth, the Fibroblast Growth Factors (FGFs) (you can know more about this condition here). One of the proposed ways of achondroplasia pathogenesis at the molecular level is that when the achondroplasia mutation is present the binding of FGFs to FGFR3 stabilizes this receptor further, making it difficult for the cell to degrade it after it was activated (this is how the cell normally terminates FGFR3's activity) [1].
What is TA-46 and what does it do?
So, instead of acting on an inhibitor molecule, like Vosoritide, which is an analog of a signalling molecule naturally occurring in the human body (CNP) that signals for the inhibition of one of FGFR3's signal cascades (learn more here), TA-46 is a soluble form of FGFR3 (sFGFR3), that captures the ligands, FGFs, before they can bind to FGFR3 [2], reducing the number of binding between FGFs and FGFR3 that exist on the cell's surface and avoiding, at least in part, the excessive activity of the receptor.
 |
 |
 |
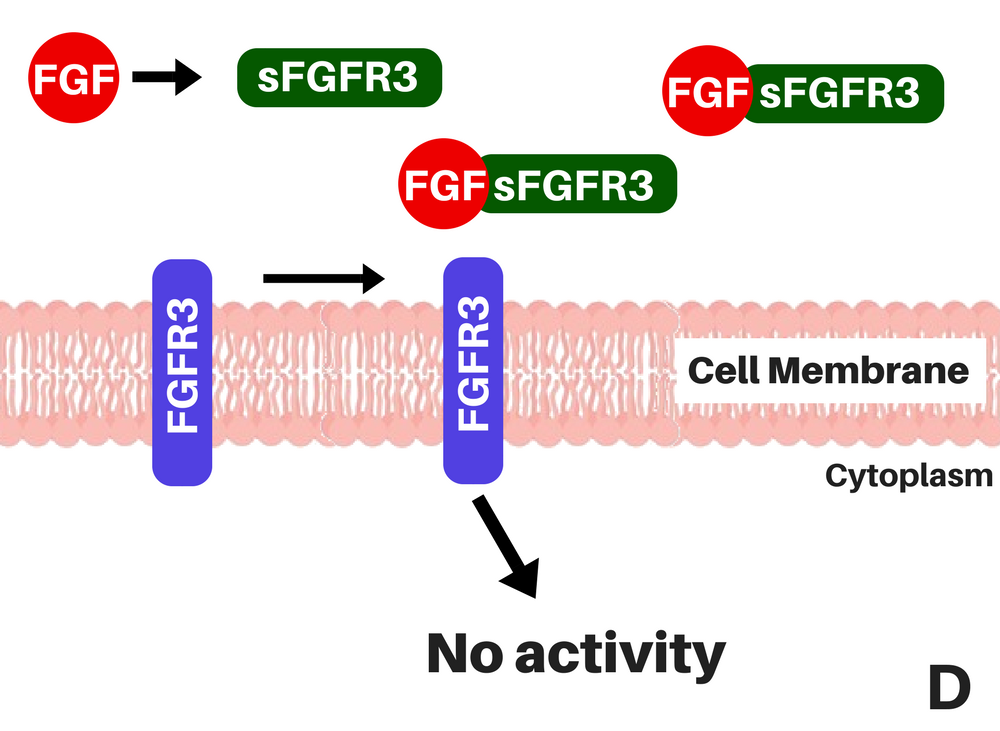 |
| Where chondrocytes are in the bone (A), how FGFR3 normally works (B), how it works with achondroplasia (C), and sFGFR3's (TA-46) mechanism of action (D). This happens at the surface of chondrocytes, in the growth plate of long bones (i.e. the femurs). Adapted from: Therachon. |
What stage of development is TA-46 in?
Pre-clinical data in the mouse model show the ability of TA-46 to restore bone growth and metabolism defects that are associated with achondroplasia. sFGFR3 has also received Orphan Drug designation by both the FDA (US Food and Drug Administration) and the EMA (European Medicines Agency). The Phase 1 clinical trial started in 2018 and the first participant has already been dosed. In Phase 1 clinical trials healthy participants are dosed with the drug (in this case TA-46), so no therapeutic effect is expected from this trial, where the objective is to assess if the drug is safe to administer in a patient and its pharmacokinetics [3].
However, in preparation for a possible Phase 2 clinical study in 2019, Therachon is planning a natural history study, which will not only increase our overall knowledge of achondroplasia, but will also allow the establishment the baseline values of general health status improvements, such as growth rate [4].
However, in preparation for a possible Phase 2 clinical study in 2019, Therachon is planning a natural history study, which will not only increase our overall knowledge of achondroplasia, but will also allow the establishment the baseline values of general health status improvements, such as growth rate [4].
Sources:
- Cho, J.Y., et al., Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proc Natl Acad Sci U S A, 2004. 101(2): p. 609-14.
- Therachon. Research. 2017 [cited 2017 25/05].
- Therachon Announces Dosing of First Subject in Phase 1 Clinical Trial Evaluating TA-46, a Novel Investigational Therapy for the Potential Treatment of Achondroplasia, Business Wire [cited 23/06].
- Therachon Announces Start of Natural History Study in Children with Achondroplasia, Business Wire [cited 23/06].


