- 27 Feb 2017 - TA 46 received Orphan Drug status for Achondroplasia by the European Medicines Agency, EMA

| Captured image - EMA documents library |
- 2 Jun 2017 - TA 46 received Orphan Drug status for Achondroplasia by the US Food and Drug administration, FDA
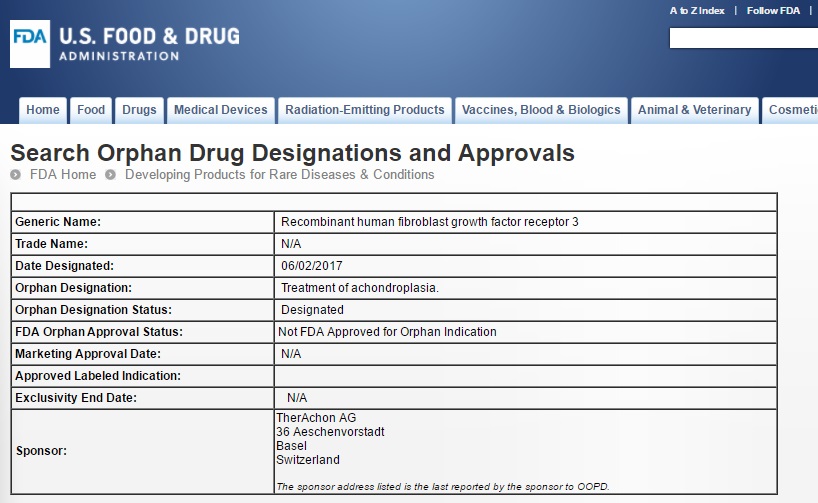
| Captured image in Access Data FDA |
- 15 Jun 2017 - Therachon signed an agreement with Catalent Pharma Solutions, to support the preclinical and clinical development of TA-46, heading to plan of clinical trials in paediatric patients with Achondroplasia
- 20 Jun 2017 - Press release of Therachon receiving orphan drug designation for TA-46
About Orphan Designation
FDA: The Orphan Drug Act (ODA) provides for granting special status to a drug or biological product ("drug") to treat a rare disease or condition upon request of a sponsor.
EMA: The European Medicines Agency is responsible for reviewing applications from companies /pharmaceutical industry, who intend to develop medicines for rare diseases, known as 'orphan drugs' (EMA, 2017). When an Orphan Drug designation is granted, EMA provides incentives for the drug's development as:
- Market exclusivity: 10 years with no competition by similar products,after the drug is approved for sale
- Protocol assistance: The agency provides scientific advice to optimize development and guidance on preparing a dossier that will meet European/US regulatory requirements
- Fee reductions
- EU-funded research
Designation as an orphan medicinal product does not indicate that the product has already satisfied the efficacy, safety and quality criteria necessary for the granting of a marketing authorization. As with any medicine, these criteria can only be assessed once the application for marketing authorization has been submitted.


