Breaking news - Preliminary Phase 1 data for TransCon CNP

| Credits - Ascendis Pharma (preliminary phase 1 data). |
Phase 1 clinical trial
It was:
Phase 1 design

| Phase 1 design - credits - Ascendis Pharma (preliminary phase 1 data) |
The trial enrolled 45 healthy adult subjects from 2 study centers in Australia, that were divided into different groups. Five doses of TransCon CNP were tested sequentially, beginning with the lowest dose: 3.0, 10, 25, 75 and 150 microgram/kg. Up to 10 subjects in each dose cohort were randomized to receive TransCon CNP or placebo in a 4:1 ratio. After each cohort completed dosing, a Data Safety Monitoring Board (DSMB) reviewed the blinded data to approve escalation to the next higher dose.
The primary endpoint was the frequency of adverse events after administration of TransCon CNP.
Secondary endpoints included additional safety parameters, tolerability, and pharmacokinetics.
Preliminary safety results of TransCon CNP
The key aspects of the data presented by Ascendis were that no serious Adverse Events or undesirable side effects were reported in the trial.
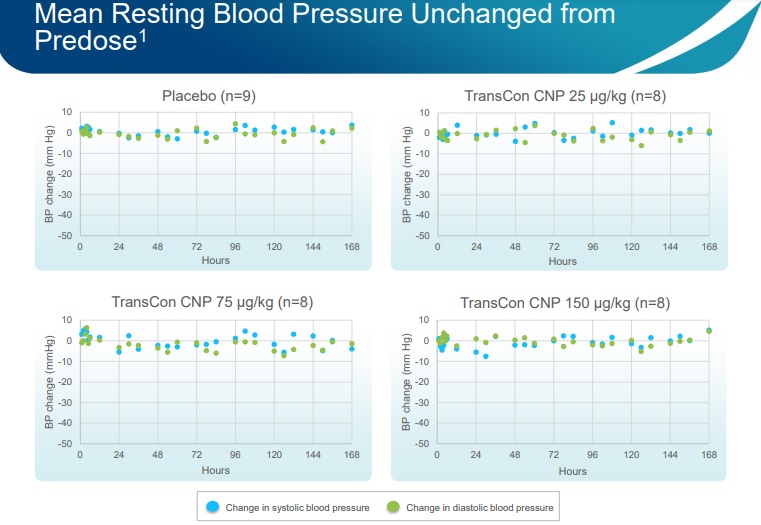
Also, the company reported that TransCon CNP was generally well tolerated at doses up to 150 µg/kg. Comparing to a similar compound, Vosoritide, now in phase 3, is being administered in a 15 µg/kg/daily, regarding the mean resting blood pressure ( that is taken when a person is not doing any activity) and heart rate were unchanged from predose at all time points, in all cohorts. This is also an important aspect, comparing with Vosoritde that produces non-serious, transient (i.e. minutes), and resolved without medical intervention reductions in the blood pressure and heart rate.
Also, Ascendis reported that injections were well tolerated in all dose cohorts with no reported injection Adverse events.
Top conclusions
1. The TransCon CNP phase 1 data reproduced Pharmacokinetics (PK) profile and cardiovascular safety from preclinical studies.
This statement means that the results now obtained are in the same line of the results previously obtained in the mouse model.
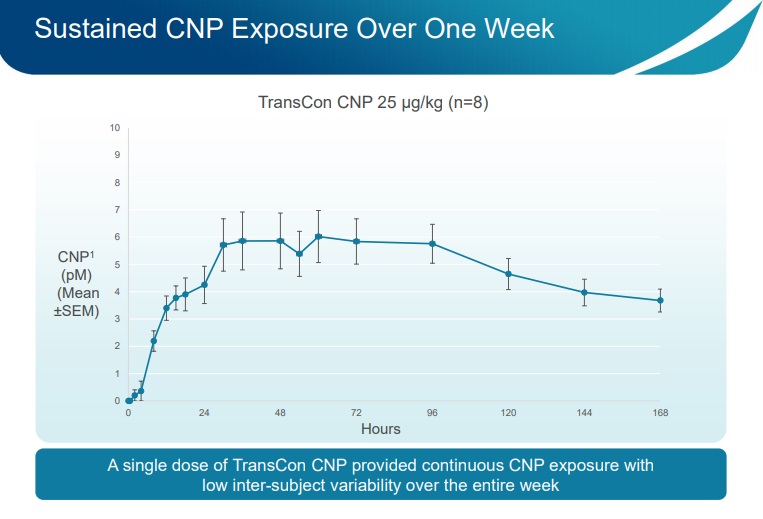
2. A single subcutaneous administration (injection of TransCon CNP)per week was confirmed based on the continuous values of CNP over seven days
3. The continuous CNP levels are important for balancing the CNP/FGFR3 pathways and normalizing growth
4. Generally well tolerated across all cohorts
5. Potential for a significant impact on patients’ lives, not only affecting height but also addressing many comorbidities associated with achondroplasia.


