A new step forward for TherAchon: the Dreambird study on Achondroplasia
| Image adapted from Brain for the cure |
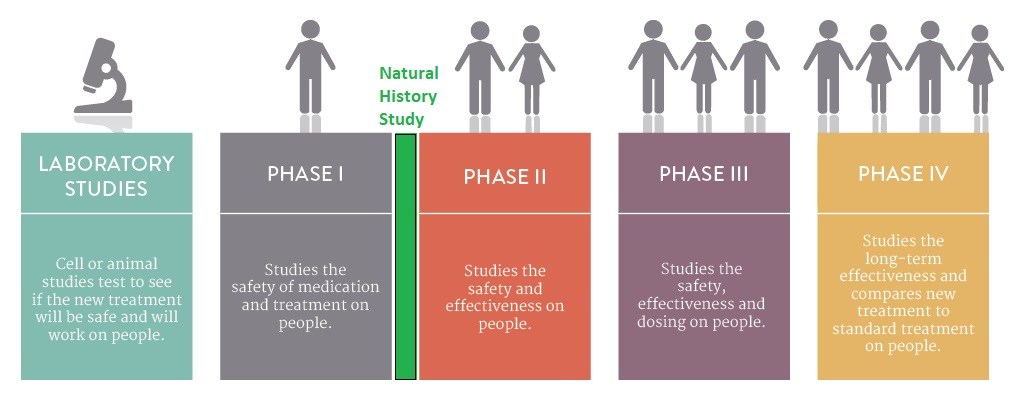
TherAchon has announced at the end of June that they have started a Natural History study on achondroplasia, called Dreambird.
The Dreambird study will follow-up 200 participants from several sites in Europe, USA and Canada which will evaluate and measure children growth, disease progression, the potential risk of complications related to ACH and protection factors, among other biological parameters for a specific period [2]. Which parameters will be measured, exactly, have not been disclosed yet, but the full details of this study will be available in the Clinical Trials website very soon.
Is important to emphasize that, because the Dreambird study is an observational study, the experimental drug TA-46 will not be administered at any time in this study.
Nevertheless, since one of the objectives of this study, besides providing a better understanding of pediatric achondroplasia, is to establish the baseline growth rates and to identify biomarkers for achondroplasia, it will be mandatory for children to participate in the Dreambird study before being enrolled in the future interventional studies, namely: the phase 2 and 3 of the TA-46 clinical trial.
The age of children to be enrolled in the Dreambird study is from 0 to 10 years old.
Sources:
- Therachon Announces Start of Natural History Study in Children with Achondroplasia, Business Wire [cited 11/07].
- Therachon starts a Natural History Study in children with achondroplasia, Fundación ALPE [cited 11/07].


