The soluble FGFR3, Therachon's TA- 46, may prevent early onset of obesity in achondroplasia
Obesity and metabolism are important topics related to achondroplasia but rarely discussed.
Developed especially during childhood, obesity is a common complication associated with achondroplasia, affecting more than 50% of people with this condition. The extra weight exacerbates many other complications associated with achondroplasia, such as lumbar lordosis and obstructive sleep apnea, and may play a role in the increased cardiovascular-related death observed in people with achondroplasia, making it a relevant medical complication in the management of this disease [1].
Developed especially during childhood, obesity is a common complication associated with achondroplasia, affecting more than 50% of people with this condition. The extra weight exacerbates many other complications associated with achondroplasia, such as lumbar lordosis and obstructive sleep apnea, and may play a role in the increased cardiovascular-related death observed in people with achondroplasia, making it a relevant medical complication in the management of this disease [1].
This complication is generally accepted as a risk factor for a number of complications, such as cardiovascular disease, diabetes, and hypercholesterolemia (excess cholesterol in the blood) in the general population. Although cardiovascular disease is higher in people with achondroplasia than in the general population [2], a few case studies suggested that achondroplasia related obesity doesn't cause alterations in insulin and cholesterol levels [3-5].
This study was first presented in the 2017 International Skeletal Dysplasia Society meeting (report of ISDS meeting here) by Dr. Celine Saint-Laurent, from Elvire Gouze's reaserch team. The team demonstrated the existence of metabolic alterations in an achondroplastic mouse model and in children with achondroplasia and also showed how the soluble FGFR3 (sFGFR3) therapy can revert these metabolic alterations in this mouse model.
By performing a longitudinal retrospective study, with the evaluation of anthropometric measures (body mass index - BMI, height, weight, etc.) and blood parameter values. These parameters were recorded and compared between children of 3 age groups: 0-3, 4-8 and 9-18 years old and the researchers discovered that glucose and insulin levels, were within normal, but that these children had a tendency to have low cholesterol and triglyceride levels, which is markedly different from what happens in obesity not associated with achondroplasia.
To understand what happens to children with achondroplasia, the research team performed a longitudinal retrospective study. So what is this?
In a longitudinal study, subjects are followed over time with continuous or repeated monitoring of risk factors or health outcomes, or both [6]. And retrospective is when a study looks backwards and examines exposures to suspected risk or protection factors in relation to an outcome that is established at the start of the study [7].

| Credits: Boston University – School of public health |
They also evaluated anthropometric measures: body mass index – BMI, height, weight, etc, and blood parameter values were recorded and compared between children of 3 age groups: 0-3, 4-8 and 9-18 years old. After this, the researchers discovered that glucose (blood sugar) and insulin levels (a hormone made by the pancreas that allows your body to use glucose from carbohydrates in the food consumed for energy or to store glucose for future use. Insulin helps keeps your blood sugar level from getting too high (hyperglycemia) or too low (hypoglycemia) [8], were within normal, but these children had a tendency to have low cholesterol and triglyceride levels (fat), which is markedly different from what happens in obesity not associated with achondroplasia.
Key points:
1. The fact that obesity in achondroplasia is different at a metabolic level from obesity in the general population and that it is a relevant medical complication makes it a good indicator for the efficacy of a medication for achondroplasia.
2. Although sFGFR3 has already shown to increase body length and decrease mortality in an achondroplasia mouse model, the researchers also measured how this drug would affect these metabolic disturbances in the same mouse model and demonstrated that the soluble FGFR3 prevented the atypical abdominal obesity observed in untreated mice and that it normalized some of the blood parameters.
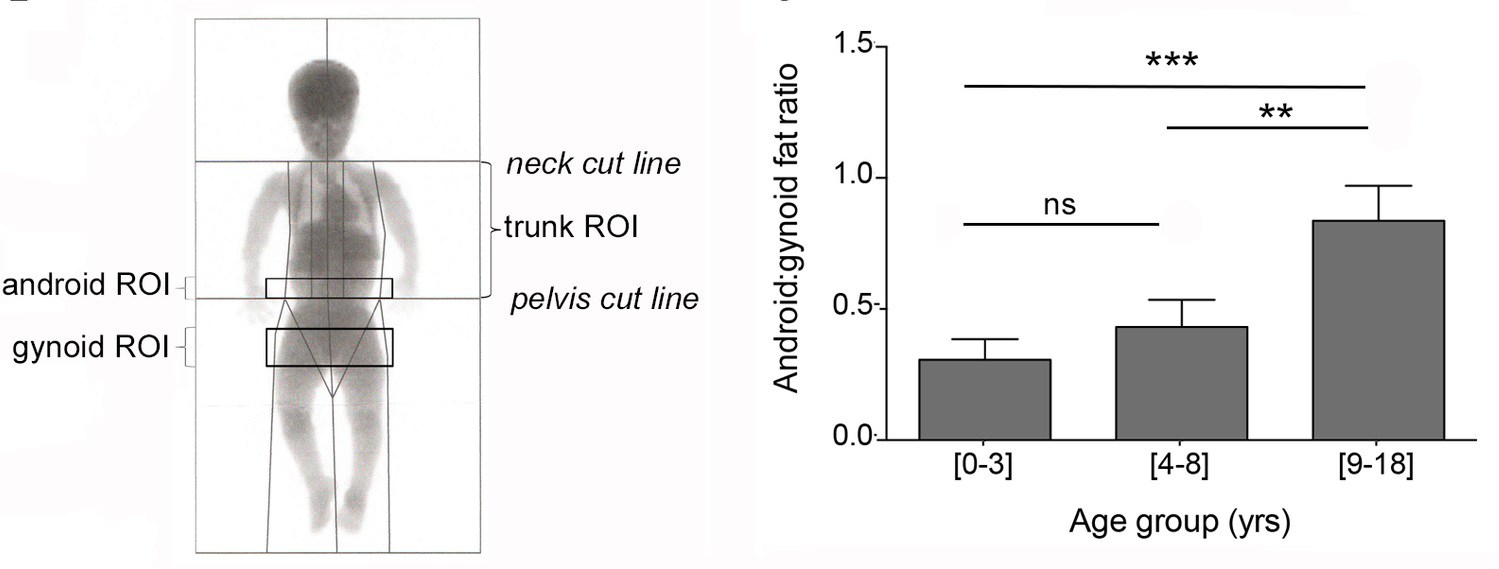
| Regions of interest (ROI) for obesity in children with achondroplasia and Android:gynoid fat ratio measurement in the three age groups. Credits: Saint-Laurent C, Garcia S et al., 2018. Android fat distribution describes the distribution of human adipose tissue in the trunk and upper body while Gynoid fat refers to the body fat that forms around the hips, breasts and thighs [10] |
This article was published on April 13th, 2018 and can be accessed for free below:
Early postnatal soluble FGFR3 therapy prevents the atypical development of obesity in achondroplasia [9]
Abstract:
Background:
Achondroplasia is a rare genetic disease is characterized by abnormal bone development and early obesity. While the bone aspect of the disease has been thoroughly studied, early obesity affecting approximately 50% of them during childhood has been somewhat neglected. It nevertheless represents a major health problem in these patients and is associated with life-threatening complications including increased risk of cardiovascular pathologies. We have thus decided to study obesity in patients and to use the mouse model to evaluate if soluble FGFR3 therapy, an innovative treatment approach for achondroplasia, could also impact the development of this significant complication.
Methods and findings:
To achieve this, we have first fully characterized the metabolic deregulations in these patients by conducting a longitudinal retrospective study, in children with achondroplasia Anthropometric, densitometric measures as well as several blood parameters were recorded and compared between three age groups ranging from [0±3], [4±8] and [9±18] years old. Our results show unexpected results with the development of an atypical obesity with preferential fat deposition in the abdomen that is remarkably not associated with classical complications of obesity such as diabetes or hypercholesterolemia. Because it is not associated with diabetes, the atypical obesity has not been studied in the past even though it is recognized as a real problem in these patients. These results were validated in a murine model of achondroplasia (Fgfr3ach/+) where similar visceral adiposity was observed. Unexpected alterations in glucose metabolism were highlighted during a high-fat diet. Glucose, insulin or lipid levels remained low, without the development of diabetes. Very interestingly, in achondroplasia mice treated with soluble FGFR3 during the growth period (from D3 to D22), the development of these metabolic deregulations was prevented in adult animals(between 4 and 14 weeks of age). The lean-over-fat tissues ratio was restored and glucose metabolism showed normal levels. Treating Fgfr3ach/+ mice with soluble FGFR3 during the growth period, prevented the development of these metabolic deregulations in adult animals and restored lean-over-fat tissues ratio as well as glucose metabolism in adult animals.
Conclusion:
This study demonstrates that:
- achondroplasia patients develop an atypical obesity with preferential abdominal obesity not associated with classical complications.
- These results suggest that achondroplasia induces an uncommon metabolism of energy, directly linked to the FGFR3 mutation.
- These data strongly suggest that this common complication of achondroplasia should be included in the clinical management of patients.
- In this context, sFGFR3 proved to be a promising treatment for achondroplasia by normalizing the biology at different levels, not only restoring bone growth but also preventing the atypical visceral obesity and some metabolic deregulations.
We look forward in seeing how this compound, the soluble FGFR3 (the same as TA-46 from Therachon) will be effective in children with achondroplasia when the company presents phase 2 clinical trial results.
Sources
- Unger, S., L. Bonafé, and E. Gouze, Current Care and Investigational Therapies in Achondroplasia. Current Osteoporosis Reports, 2017. 15(2): p. 53-60.
- Wynn, J., et al., Mortality in achondroplasia study: A 42-year follow-up. American Journal of Medical Genetics Part A, 2007. 143A(21): p. 2502-2511.
- Collipp PJ, Sharma RK, Thomas J, Maddaiah VT, Chen SY. Abnormal glucose tolerance in children with achondroplasia. Am J Dis Child. 1972; 124(5):682±5.
- Pirgon O, Atabek ME, Sert A. Achondroplasia associated with metabolic syndrome: patient report. J Paediatr Child Health. 2008; 44(10):602±4.
- Alatzoglou KS, Hindmarsh PC, Brain C, Torpiano J, Dattani MT. Acanthosis nigricans and insulin sensitivity in patients with achondroplasia and hypochodroplasia due to FGFR3 mutations. J Clin Endocrinol Metab. 2009; 94(10):3959±63.
- Coggon, D., et al. Chapter 7. Longitudinal studies. Epidemiology for the uninitiated. 2018.
- . "Prospective vs. Retrospective Studies." Retrieved 03/05, 2018.
- Hess-Fischl, A. (2017). "What is Insulin?" Diabetes. Retrieved 03/05, 2018.
- Saint-Laurent C, Garcia S et al., Early postnatal soluble FGFR3 therapy prevents the atypical development of obesity in achondroplasia, PLos One, 2018 Apr 13;13(4):e0195876.
- . "gynoid fat distribution." Oxford Reference. Retrieved 08/05, 2018.


