Vosoritide for Achondroplasia - Research and Development day 20th April 2016
On the 20th April at 8am, NY time, Sarah Noonberg, MD, PhD, Group Vice President, Head of Global Clinical Development, presented the updates for Vosoritide.
List of presented updates:
- 10 children, aged 6 to 11, received for 12-month a dose of 15 µg/kg/day (cohort 3)
- Cohort 1 and 2 (12 children), received doses 2,5 and 7,5 µg/kg/day and after the first report of 6-months of data, switched to the 15 µg/kg/day dose.
- Preliminary safety data on 30 µg/kg/day dose (cohort 4, n=9)
- Phase 3 clinical development plans
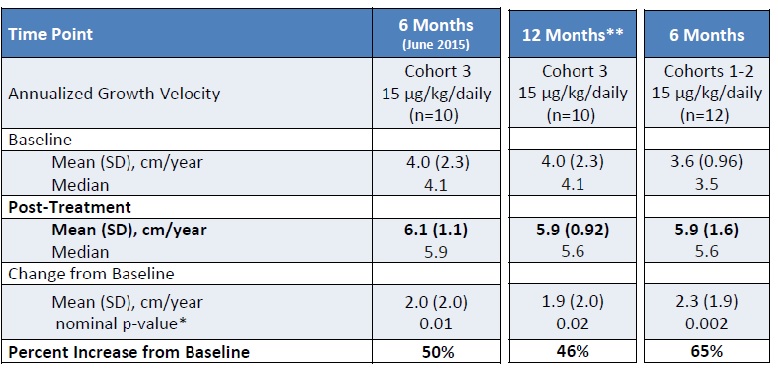
Translating this chart:
- Children from group 3, received a dose of 15 µg/kg/day, for 6 months. After this period of time, the children had grown in average 1,1 cm plus the natural growth, meaning that the predicted growth for 12 months would be 2 cm, on average, plus the natural growth (4cm/year average), in a total average of 6cm/year.
- Children from this same group 3, received the same dose for 6 more months, for a total of 12 months at this point. On average, the 10 children grew 1,9 cm plus the natural growth. BioMarin makes a remark that one child in this group skipped the majority of the injections, so the average result could be better. So in one year, the results presented are an increase of 1,9 cm/year in 10 children with a 15 µg/kg/day.
- 12 children in groups 1 and 2, had grown naturally in average, 3,6 cm/year and received lower dose for the first 6 months, and 15 µg/kg/day for other 6 months. These children have grown in average 2,3 cm/year plus natural growth.
The lower body segment is the length from the pubic to the floor; the upper body segment is the height minus the lower body segment. The U/L ratio (upper body segment : lower body segment ratio) at birth is about 1.7; at age 3 years it is 1.3; at greater than 7 years, it is 1.0 with the upper body segment and lower body segment being about equal. Higher U/L ratios are noted in short-limb dwarfism.
So the ideal is a ratio near 1.
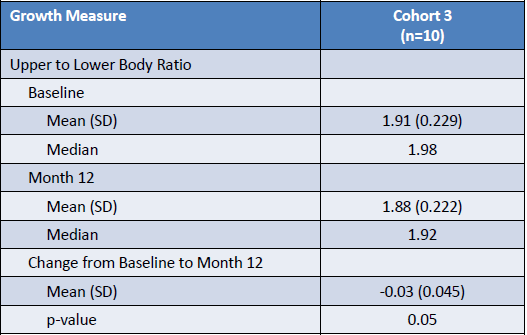
- The results BioMarin presented show small favorable changes in proportionality, with a decrease in U/L ratio although there is the need of longer treatment and/or control arm required to confirm treatment effect, as stated. So a decrease in 0,03 points in the ratio is a small difference but promising one.
- Also, in 12 months of treatment, BioMarin stated no evidence of reduction of pharmacological activity of vosoritide over 12 months based on an urinary biomarker.
- No treatment-related serious adverse events, severe adverse events or adverse events leading to dose reduction or discontinuation. The most common treatment-related adverse events were injection site reactions (mild, as stated and in 90% of subjects) and asymptomatic hypotension (40% of subjects).
- 15 µg/kg/day of vosoritide remains generally well tolerated.
- Preliminary safety data appears favorable at 30 µg/kg/day
Phase 3 Development Plans
- Planning to start phase 3 at the end of 2016
- Group of ages: 5 to 14, consistent with phase 2 population
- Primary endpoint: Annualized growth velocity
- Ongoing discussions with global health authorities - it is a good step to work in advance with the FDA and EMA.
- Additional phase 2 study will evaluate effect of vosoritide in infants/toddlers - this is when children under 5 will get an opportunity in this clinical trial.
Data from BioMarin's presentation of 20th April on the R&D Day 2016
It would be good to see data for the foramen magnum, since an increase in diameter is highly desirable.


