BMN-111 Mechanism of Action
Although achondroplasia patients are still limited to being treated for the complications that arise from their condition, instead of the condition itself, several promising orphan drugs (that target rare diseases) are being developed for the treatment of this genetic disorder [1].
BioMarin's BMN-111 (also known as Vosoritide) is opening a new road by having the first potential treatment for ACH reaching a clinical trial, which in January 2017 entered into Phase III. At the same time, other companies, such as Therachon AG, are developing other drugs to tackle this disease in other ways.
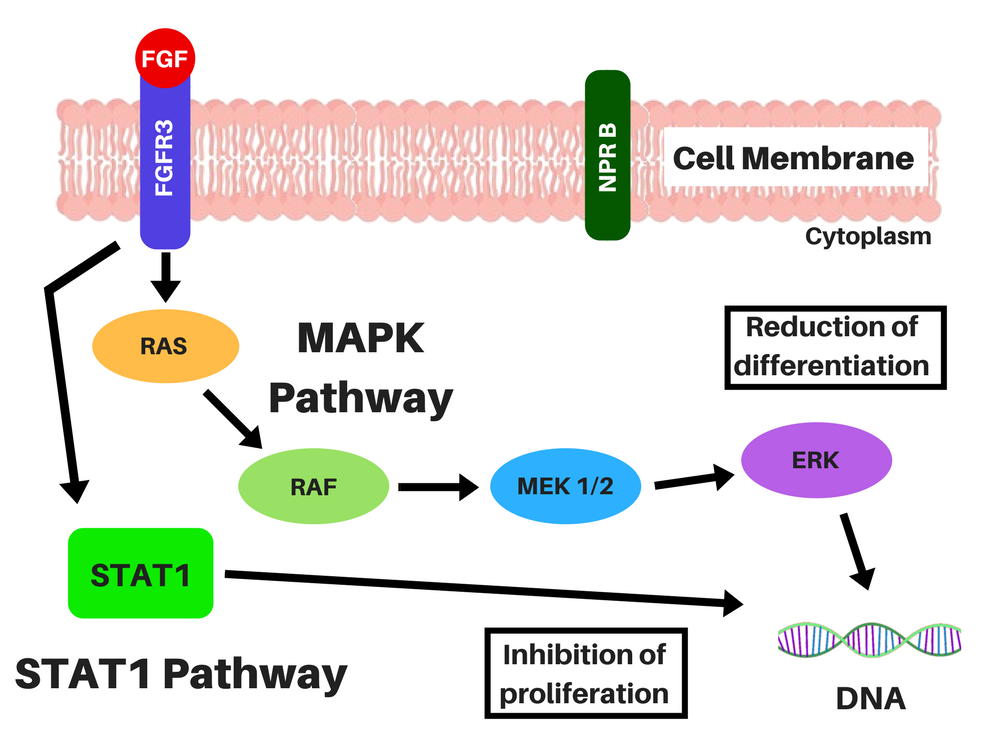
Achondroplasia is caused by a single point mutation in the gene that encodes for Fibroblast Growth Factor Receptor 3 (FGFR3), causing it to be constitutively activated (you can know more about this condition here). This permanent activation of FGFR3 signaling activates two intracellular signaling cascades that lead to a lower proliferation and differentiation of bone growth plate chondrocytes, through the STAT-1 pathway, and to a lower production of extracellular matrix (ECM) through the MAPK pathway [2]. Basically, it slows growth in two different ways, through two mechanisms.
What is BMN-111 and how does it work?
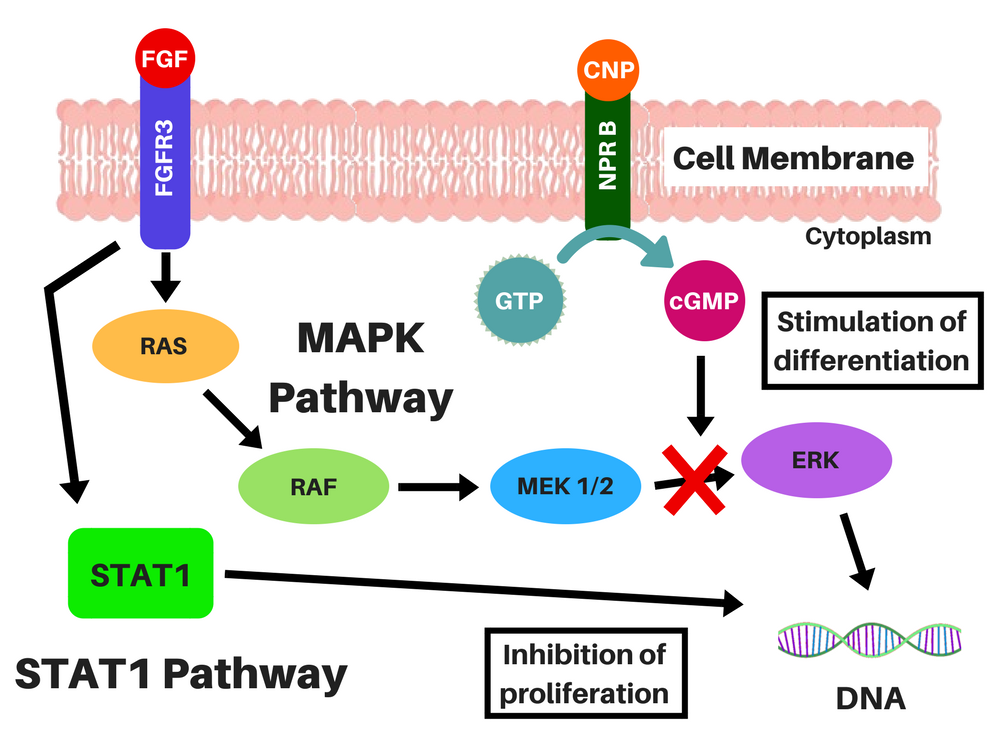
BMN-111 is an analog of a naturally existing peptide, the C-type Natriuretic Peptide (CNP), with a longer half-life (it is resistant to digestion by neutral-endopeptidase, an enzyme that digests other proteins) [3]. This peptide binds to the Natriuretic-Peptide Receptor B (NPR B), which induces the synthesis of cyclic guanosine-3′,5′-monophosphate (cGMP) molecules, which, in turn, inhibit the MAPK pathway (by inhibiting certain enzymes in this pathway) [2, 4, 5].
 |
 |
 |
|
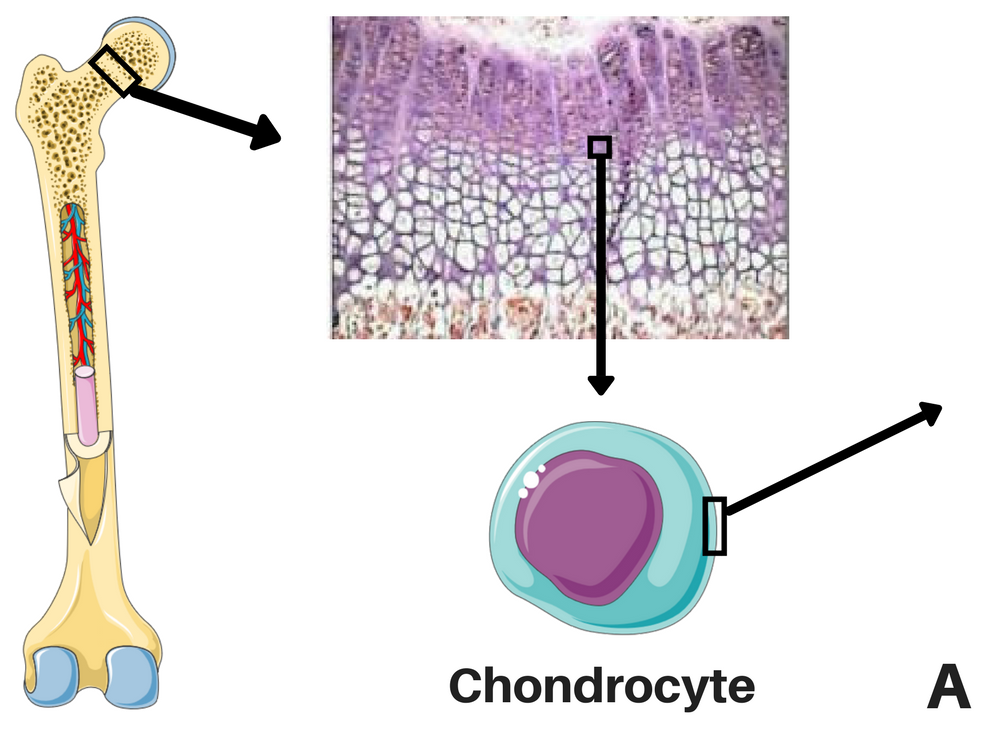
Location of the chondrocytes (left) and representations of FGFR3 signaling without CNP (middle), and CNP and vosoritide's action within a cell (right). STAT-1 pathway lowers proliferation and differentiation of chondrocytes and MAPK pathway lowers ECM synthesis through a complex cascade and reduces differentiation of chondrocytes. CNP induces the production of cGMP, which will inhibit the action of MEK1/2 in the MAPK pathway. Credits: adapted from Morrys C. Kaisermann. |
Even though BMN-111 affects the MAPK pathway, it does not affect the STAT-1 pathway (which is the signal the drugs under development by Therachon AG will also try to block), and there is a possibility that people with achondroplasia, hypochondroplasia and thanatophoric dysplasia may be resistant to naturally existent CNP [2, 6, 7].
What effects has BMN-111 been shown to have?
BMN-111 has shown to reduce the achondroplastic phenotype, improving the short stature of patients with achondroplasia in the Phase II clinical trial, only mild adverse effects, such as: injection site reactions, headaches, hypotension, back pain and cough [8]. However, there's still no data on long-term efficacy of this drug, about its side effects or about how it affects proportionality.
How far in development is it?
This drug is now in Phase III clinical trial and a global Phase II study for children under 5 years of age has started in 2018 and is ongoing. The first participant for the Phase 2 clinical trial in children has already been dosed with this drug [9].
Sources:
- LEVITEN, M. COMPETING FOR GROWTH. TARGETS & MECHANISMS 2017 [cited 2017 15/06].
- Yasoda, A., et al., Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med, 2004. 10(1): p. 80-86.
- Lorget, F., et al., Evaluation of the Therapeutic Potential of a CNP Analog in a <em>Fgfr3</em> Mouse Model Recapitulating Achondroplasia. The American Journal of Human Genetics. 91(6): p. 1108-1114.
- Peake, N.J., et al., Role of C-type natriuretic peptide signalling in maintaining cartilage and bone function. Osteoarthritis and Cartilage, 2014. 22(11): p. 1800-1807.
- Wu, Y., Chen, Y., Qu, R., Lan, T., & Sang, J. (2012). Type II cGMP-dependent protein kinase inhibits EGF-triggered signal transduction of the MAPK/ERK-mediated pathway in gastric cancer cells. Oncology Reports, 27, 553-558.
- Olney, R.C., et al., C-type natriuretic peptide plasma levels are elevated in subjects with achondroplasia, hypochondroplasia, and thanatophoric dysplasia. J Clin Endocrinol Metab, 2015. 100(2): p. E355-9.
- Wendt, D.J., et al., Neutral Endopeptidase-Resistant C-Type Natriuretic Peptide Variant Represents a New Therapeutic Approach for Treatment of Fibroblast Growth Factor Receptor 3–Related Dwarfism. Journal of Pharmacology and Experimental Therapeutics, 2015. 353(1): p. 132.
- BioMarin Presents Vosoritide Data in Achondroplasia at American Society of Human Genetics (ASHG) 2016 Meeting, in GlobeNewswire2016, Nasdaq.
- BioMarin Doses First Participant in Phase 2 Study of Vosoritide for Treatment of Infants and Young Children with Achondroplasia, Biomarin Press Release [cited 23/06/2018]


