Industry
Ascendis Pharma
Ascendis Pharma is developing TransCon CNP, which had positive results in pre-clinical studies. The company aims to release new data on a phase I clinical trial in the fourth quarter of 2018.
Visit their website.
BioClin Therapeutics, Inc
Pipeline

|
Adapted from: BioClin Therapeutics (08/2017)
|
Visit their website.
BioMarin
Pipeline
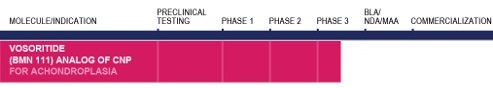
| Adapted from: BioMarin (08/2017) |
Visit their website.
Ribomic
Pipeline
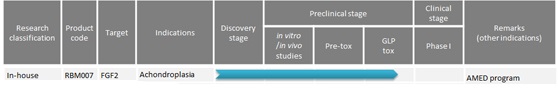
| Adapted from: Ribomic (09/2017) |
Visit their website.
Therachon
Therachon is developing TA-46, which has already acquired Orphan Drug status and is heading towards phase II clinical trial, which is planned for the beginning of 2019. The company has also announced that they are developing another drug for achondroplasia called TA-100.



