Study of Infigratinib in Children With Achondroplasia
6th March 2020
The Propel study aims to learn more about the overall health, growth, and possible medical complications in children with achondroplasia.
200 boys and girls are still being recruited to participate in the study at locations worldwide. No medication will be administered; however, children successfully enrolled in PROPEL for 6 months or more may be eligible for the Study of Infigratinib in Children With Achondroplasia - Propel 2.
PROPEL 2 is a multicenter, open-label, dose-escalation and dose-expansion study to evaluate the safety, tolerability, and efficacy of infigratinib, a fibroblast growth factor receptor (FGFR) 1-3-selective tyrosine kinase inhibitor, in children 3 to 11 years of age with Achondroplasia (ACH) who previously participated in the PROPEL study for at least 6 months. The study includes dose escalation with extended treatment and dose expansion.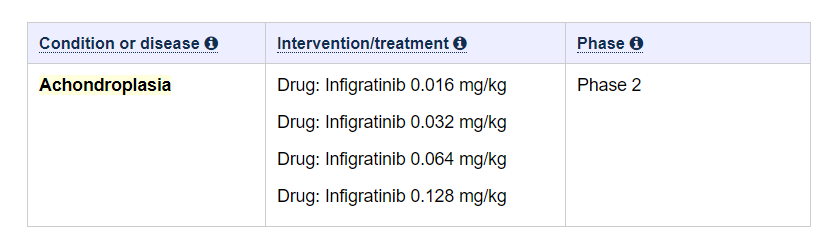
Image credits: clinicaltrials.gov
The safety and efficacy of infigratinib have not been established and there is no guarantee that infigratinib will receive health authority approval or become commercially available in any country for the uses being investigated.


