[Free Full Text] The activity of FGFR3 with the achondroplasia mutation
Science is dynamic and new discoveries are emerging everyday. New advances on the activity of FGFR3, the receptor that has a mutation in achondroplasia, have been published.
FGFs (fibroblast growth factors) and their receptors (FGFRs) play essential roles in tightly regulating cell proliferation, survival, migration and differentiation during development and adult life.
The FGFRs have an overall structure similar to most RTKs. They are single-pass transmembrane proteins that consist of an extracellular part that binds FGF ligands, a transmembrane domain and an intracellular tyrosine kinase domain that transmits the signal to the interior of the cell. In Fibroblast growth factors and their receptors in cancer,
The Receptor Tyrosine Kinases (RTK) is a group of cell-surface receptors where FGFR3 is included, and represent an important family of genes that is commonly affected by mutations and alterations in human cancers. All RTK share the same composition: an extracellular ligand-binding region, a single-pass transmembrane domain and an intracellular tyrosine kinase domain.

| Figure 1 - Credits: "Fibroblast growth factors and their receptors in cancer"- Biochemical journal, 2011. |
The complex formed by 2 FGFs + 2 FGFRs (plus 2 heparan sulfate chains) causes dimerization and a chain reaction inside the cell, designated downstream signalling cascades. The FGFR3 dimerization forms a dimer (a chemical structure formed from two similar sub-units). The ACH mutation occurs in one portion of the FGFR3, the transmembranar domain.
The dimerization concept might be easier to visualize with the following example: chopsticks.
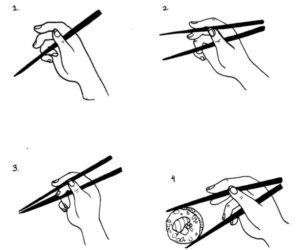
| Image credits. |
Imagine that each portion of FGFR3 is one chopstick. When one person holds two chopsticks can produce a movement to get the tips together. Grabbing food with chopsticks is just possible because there are two. With just one, that would not be possible.
A similar action happens when FGFR3 is activated: the two parts of the FGFR3 get together when a ligand binds (going back to eating with chopsticks, the ligands would be associated to the hand that holds them).
Supposedly, the FGFR3 would only form a dimer and start the intracellular chain reaction after the binding of ligands, as proposed in Figure 1. But the ACH mutation causes a structural change in FGFR3 that affects the stability and activity of FGFR3 dimers even in the absence of ligand. In "FGFR3 mutation causes abnormal membranous ossification in achondroplasia", Legait Mallet et al. Human Molecular Genetics, 2014
In 2014, Hristova et al., published "Dimerization of FGFR3 in Living Cells", Biophysic Journal. The research team from Johns Hopkins University, developed a FRET-based methodology (Förster/Fluorescence resonance energy transfer) to measure the dimerization of RTKs in cells.
Recently, Sarabipour S. and Hristova K., published "Effect of the achondroplasia mutation on FGFR3 dimerization and FGFR3 structural response to fgf1 and fgf2: A quantitative FRET study in osmotically derived plasma membrane vesicle" in Biochim Biophys Acta., July 2016.
In relation to her studies, Prof. Kalina Hristova was kind to reply to some questions.
Questions and answers - Beyond Achondroplasia and Prof. K. Hristova
BA - Can FGFR3 dimerize and start the tyrosine kinase downstream cascade with and without ligands?
KH- This is correct, but the activity with and without ligand is different. This is because the dimer undergoes structural changes when the ligand binds and activity increases. Also note, we have not looked at downstream signaling, just FGFR3 kinase phosphorylation.
BA- The concept that FGFR3 would only dimerize and phosphorilate after one ligand binded is not correct. When the FGFR3 has the ACH mutation, the dimerization is more stable and lasts longer. You stated that FGFR3 +ACH dimerizes and phosphorilates even without ligands. And that this activity of the dimerized (active) FGFR3+ACH after NO ligands binding is also more stable than the FGFR3 with no ligand binding. Can you confirm this?
KH - Yes, the ACH mutation stabilizes the unligated dimer
BA - Did you conclude what the percentage of dimerized FGFR3+ACH no ligand binding versus the dimerized FGFR3+ACH ligand binding was?
KH - This will depend on the expression. I can calculate all this if I know the relevant expression level of FGFR3 in chondrocytes. I have been trying to find these values in the literature.
BA - If the FGFR3+ACH acts without ligands, is an advantage of capturing ligands (FGF's) in the intercellular space?
KH - This should be valid. For the WT (wild type of FGFR3 with no mutation) and the ACH mutant, the ligand causes a structural change which increases activity.
So the reality in ACH is this: Ligand independent and dependent activation.

| Figure 2 - Credits: "Fibroblast growth factors and their receptors in cancer"- Biochemical journal, 2011. |
If FGFR3 can produce a reaction inside the cell without ligands (this, in a very imaginative way would be like eating sushi with chopsticks without using a hand...), most probably, a potential treatment for ACH that can act directly on the FGFR3 has a higher chance of being effective.
Dr. Kalina Hristova holds a joint appointment in biomedical engineering at the Johns Hopkins University School of Medicine. She is a professor and the Marlin U. Zimmerman Jr. Faculty Scholar at the Johns Hopkins University Whiting School of Engineering. Dr. Hristova received her B.S. and M.S. from the University of Sofia in Bulgaria. She earned her Ph.D. at Duke University.


